𝕋𝕙𝕖 𝔻𝕦𝕒𝕝 ℕ𝕒𝕥𝕦𝕣𝕖 𝕠𝕗 𝕃𝕚𝕘𝕙𝕥
𝔼𝕩𝕡𝕝𝕠𝕣𝕚𝕟𝕘 𝕥𝕙𝕖 𝔻𝕦𝕒𝕝 ℕ𝕒𝕥𝕦𝕣𝕖 𝕠𝕗 𝕃𝕚𝕘𝕙𝕥.
➜ Introduction:
Have you ever wondered what light really is? Is it a wave or a particle? Well, the truth is that light is both! This incredible characteristic of light is known as its dual nature. Let's dive into the fascinating world of light and explore how it can behave as both a wave and a particle.
The journey to comprehend the dual nature of light began in the 17th century when Sir Isaac Newton proposed that light consists of tiny particles called corpuscles. His corpuscular theory explained phenomena such as reflection and refraction but failed to account for the phenomena of interference and diffraction, which were later observed and explained using wave theory.
➜ Wave Theory of Light:
In the 19th century, Thomas Young conducted his famous double-slit experiment, which provided strong evidence for the wave-like nature of light. Young's experiment showed that when light passes through two narrow slits, an interference pattern is formed on a screen, indicating that light waves can interfere constructively and destructively, just like other types of waves.Further experiments, including Augustin-Jean Fresnel's work on diffraction, confirmed the wave-like behavior of light. The wave theory successfully explained phenomena like interference, diffraction, and polarization, solidifying the understanding of light as an electromagnetic wave.
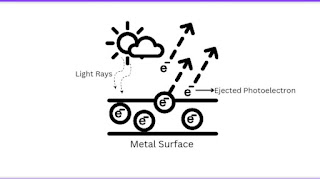
➜ The Photoelectric Effect:
In the early 20th century, the dual nature of light took a revolutionary turn with the discovery of the photoelectric effect. Albert Einstein, building upon the work of Max Planck, proposed that light consists of discrete packets of energy called photons. The photoelectric effect, observed when light strikes a metal surface, showed that electrons are emitted only when the light's energy exceeds a certain threshold, irrespective of its intensity.The photoelectric effect challenged the wave theory of light, as the wave model could not explain why the emission of electrons depended on the light's energy rather than its intensity. Einstein's explanation, considering light as a stream of particles (photons), provided a breakthrough in understanding the particle-like behavior of light.
➜ Wave-Particle Duality:
The concept of wave-particle duality, which emerged from the understanding of light's dual nature, states that particles like light can exhibit both wave-like and particle-like characteristics. This principle extends beyond light to other particles, such as electrons and even larger particles like atoms and molecules.The wave-particle duality of light is not a contradiction but a reflection of the intricate nature of the quantum world. It is described by the mathematical framework of quantum mechanics, where particles are represented by wave functions that evolve and exhibit wave-like and particle-like behavior depending on the specific experimental conditions.
➜ Applications and Impact:
The recognition of light's dual nature has revolutionized various scientific disciplines. In the field of optics, it has led to advancements in technologies such as lasers, fiber optics, and holography, transforming communication, imaging, and data storage.Furthermore, the understanding of the dual nature of light paved the way for the development of quantum mechanics, a cornerstone of modern physics. Quantum mechanics has profoundly impacted fields like quantum computing, quantum cryptography, and precision measurements.
➜ Conclusion:
The concept of the dual nature of light has reshaped our understanding of the physical world. Light, once thought to be a simple wave or particle, reveals its complex nature when investigated closely. Its ability to exhibit both wave-like and particle-like behavior challenges our classical intuitions and opens doors to new scientific discoveries and technological innovations. By embracing the dual nature of light, we embark on a journey to unravel the mysteries of the universe and deepen our understanding of the fundamental building blocks of nature.


Comments
Post a Comment